imuno®/GcMAF Patient Monitoring
GcMAF/imuno® has been used, with positive results, in many types of cancers at all stages. However, the challenge is to accurately monitor patient progress to ensure health is improving.The Ruggiero Treatment Evaluation Scale (RTEC)
imuno® works on the immune-neuro-endocrine axis as an adaptogen and, therefore, it may rebalance a number of functions that pertain to the spheres of neurology, psychiatry, psychology, and cognition. These effects are of importance not only for those conditions where symptoms pertaining to these spheres are prominent such as, for example, neuroborreliosis, chronic fatigue syndrome or neurodegenerative disease, but also for cancer.In fact, diagnosis and treatment of cancer is known to influence psychological well-being to a significant degree. Rates of psychological distress are elevated for most individuals who have been diagnosed with cancer when compared to population norms. Common psychological reactions to cancer are mood and anxiety-related concerns. Elevated rates of depression and anxiety in response to a cancer diagnosis is often attributable to uncertainty regarding mortality and well as going through arduous treatments and concerns related to functional interference and body-image or other self-concept related distress. Understanding how individuals react psychologically to cancer is important to support their overall well-being and maximize the quality of life during treatment and beyond. While the prevalence of psychological disturbance in reaction to cancer is relatively high when compared to population norms, many individuals report fairly stable psychological well-being through the cancer trajectory and some even report improved psychological wellbeing.
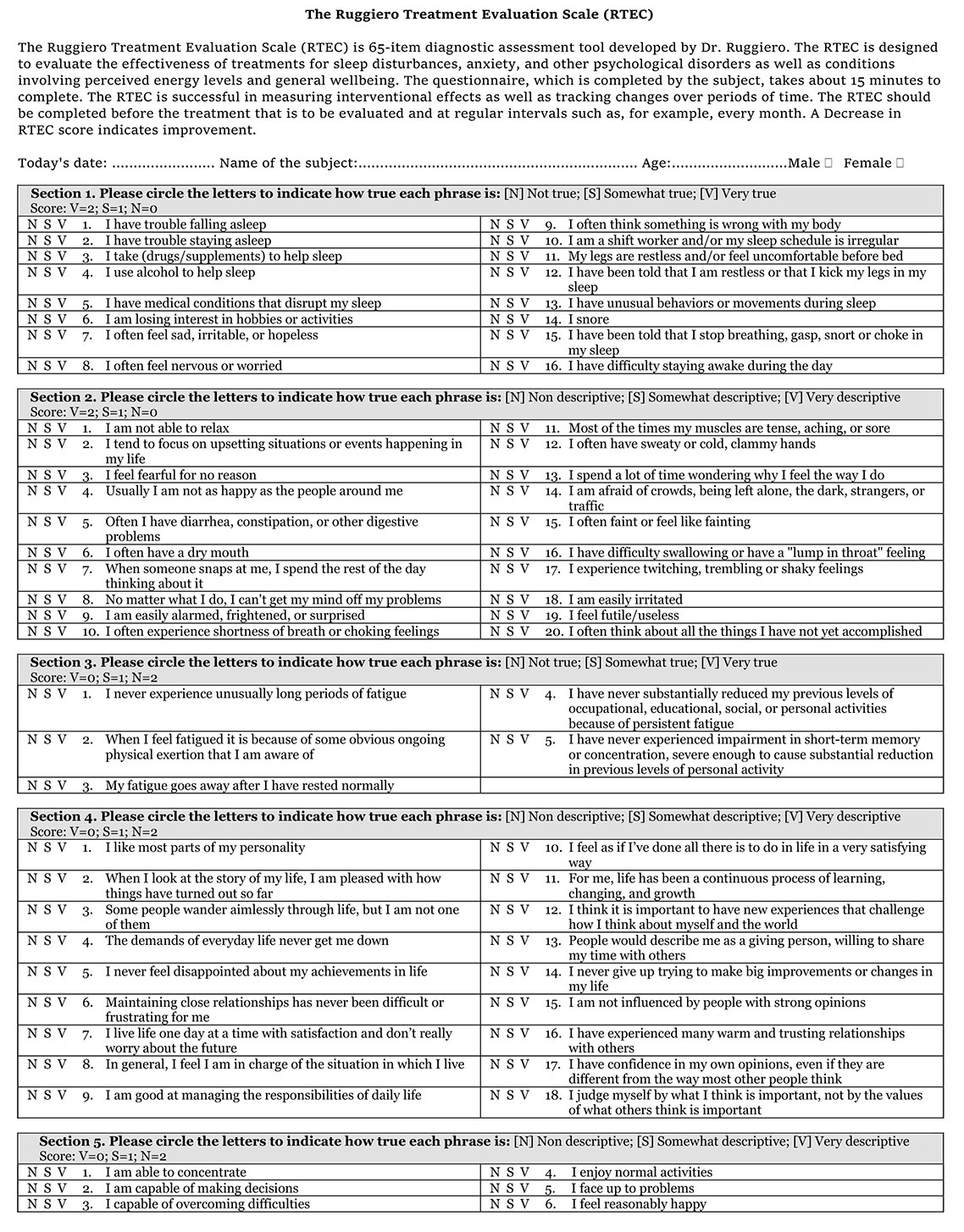
In order to evaluate the effects of imuno® on these states, a dedicated questionnaire termed "The Ruggiero Treatment Evaluation Scale (RTEC)" has been developed with the goal of evidencing those effects of imuno® that may escape the attention of the Therapist focused primarily on the specific symptoms of the disease for which imuno® is used.
It is worth noticing that the effects of imuno® on the immune-neuro-endocrine axis may be slow and progressive; since imuno® works by rebalancing physiological mechanisms these effects may go unnoticed unless specifically addressed. The RTEC has been developed precisely to address these aspects and provides a useful tool to assess the efficacy of the treatment in addition to the specific analyses or lab test that evaluate the primary disease.
The RTEC has to be compiled by the patient, not the Therapist. First assessment has to be performed before starting the treatment with imuno® and at regular intervals thereafter. As a rule of thumb, the second assessment should occur not earlier than eight weeks after starting the treatment.
Download PDF
Scans
Scans that track tumour size are the most reliable indicator of tumour burden. Ultrasound is non-toxic and accurate but cannot always find the lesions, so is good for monitoring an indicative tumour for response to treatment, but not so good for full body monitoring. A Magnetic scan (MRI) is accurate and quite safe but does not image the lungs well. A Radiation scan (PET with radioactive glucose tracer) is the gold standard for imaging cancer in the body as the tracer accumulates in cancer cells due to their increased requirment for glucose. PET scans should however be limited in number, due to concerns over radiation poisoning.Conventional Markers
Conventional cancer markers (CA125, AFP, PSA, etc) in the blood may continue to be used as indicators of cancer burden.TKTL1 and Apo10 scores
TKTL1 plays a crucial role in ovarian cancer metabolism and its expression predicts poor prognosis (1); therefore, a decrease in the expression of TKTL1 may be interpreted as a sign of decreased aggressiveness of cancer itself. It should be noticed, however, that TKTL1 expression is not unique for ovarian cancers and it appears that TKTL1 belongs to a group of metabolic genes involved in the glycolytic pathway that is significantly up-regulated in a variety of tumor cells in cancer patients and plays active roles in tumor progression (2). The cumulative TKTL1 score after treatment with imuno® is expected to decrease.Apo10 is a marker of abnormal apoptosis and proliferation and it represents an independent marker for poor survival for certain carcinomas (3). Consistent with these observations, it has been recently proposed that overcoming drug resistance of Apo10-positive cells in precursor lesions and tumors by natural compounds may act as sensitizers for apoptosis or could be useful for chemoprevention (4). The cumulative Apo10 score after treatment with imuno® is expected to decrease.
Nagalase Testing
We now know that Nagalase does not accurately reflect tumor burden. It is however, a marker of chronic inflammation and so it is still useful to determine severity and progress (5).Testing in the USA
Health Diagnostics and Research Institute. New Jersey
Testing in Europe
World Health Laboratories. Netherlands
R.E.D. Laboratories. Belgium
A normal healthy Nagalase result is under 0.62, whereas a reading of 7 is very high.
In general, a cancer patient severity rating based on the Nagalase result is ...
- 0.62 to 2 minor
- 2 to 3 moderate
- 3 to 5 major
- 5 to 7 severe
Monocyte Count
A patients monocyte count will generally rise in the early stages of GcMAF/imuno® treatment and indicates a response to GcMAF/imuno®.Note: Monocytes in the blood vessels become Macrophages in the tissues.
PINI Score
The prognosis for all types of cancers is dependent upon the nutritional and inflammatory status of the patient and that can be monitored by the Prognostic Inflammatory and Nutritional Index (6).PINI Calculated by dividing the product of serum alpha-1-glycoprotein and CRP levels by that of albumin and pre-albumin.
alpha-1-glycoprotein (mg/dl) x CRP (mg/dl)
albumin (g/dl) x prealbumin (mg/dl)
albumin (g/dl) x prealbumin (mg/dl)
| > 30 | = | Predicts a very high risk of complications |
| [21-30] | = | Predicts a high risk of complications |
| [11-20] | = | Predicts an intermediate risk |
| [1-10] | = | Predicts a low risk |
| < 1 | = | Normal |
Your doctor can organise the blood tests required for the PINI score.
Ultrasonography of the spleen with echo-color-Doppler
Activation of immune cells can be assessed by evaluating vasodilation in the speenic and renal systems due to release of nitric oxide. This criterion is useful to evaluate the immediate action of the GcMAF/imuno® treatment as well as the function of the immune system which declines with age and in the presence of chronic diseases. This procedure is safe, rapid and inexpensive and has been validated in a number of papers (7).Vitamin D3
We advise keeping blood levels of vitamin D3 at between 150 to 250 nmol/l (60 ng/ml to 100 ng/ml). This can be achieved by adults taking 10,000IU of vitamin D3 daily. A blood test every 3 months is also recommended to ensure blood levels do not go above our recommended levels. Note: Vitamin D3 levels drop quickly without supplementation and high vitamin D3 levels help slow cachexia.References:
- Expression of transketolase-like 1 protein (TKTL1) in human endometrial cancer. Krockenberger M, Engel JB, Schmidt M, Kohrenhagen N, Häusler SF, Dombrowski Y, Kapp M, Dietl J, Honig A. May 2010, Anticancer Research, pp. 1653-1659.
Link to Article
- Metabolic genes in cancer: their roles in tumor progression and clinical implications. Furuta E, Okuda H, Kobayashi A, Watabe K. April 2010, Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, pp. 141-152.
Link to Article
- A biomarker based detection and characterization of carcinomas exploiting two fundamental biophysical mechanisms in mammalian cells. Grimm M, Schmitt S, Teriete P, Biegner T, Stenzl A, Hennenlotter J, Muhs HJ, Munz A, Nadtotschi T, König K, Sänger J, Feyen O, Hofmann H, Reinert S, Coy JF. December 2013, BMC Cancer, pp. 1-18.
Link to Article
- Evaluation of a biomarker based blood test for monitoring surgical resection of oral squamous cell carcinomas. Grimm M, Kraut W, Hoefert S, Krimmel M, Biegner T, Teriete P, Cetindis M, Polligkeit J, Kluba S, Munz A, Reinert S. July 2015 & March 2016, Clinical Oral Investigations, pp. 329–338.
Link to Article
- Gc Protein-Derived Macrophage Activating Factor (GcMAF) and Autism: Do Clinical Results Require a Novel Interpretation? Ruggiero, M. Schwarzenbruck. 13 Oct 2016, American Journal of Immunology, pp. 77-82.
Link to Article
- Role of Angiotensin-Converting Enzyme and Vitamin D Receptor Gene Polymorphisms in Cancer Anorexia-Cachexia Syndrome. Fabris A, Biagioni P, Punzi T, Morucci G, Gulisano M, Pacini S, Ruggiero M. 2 Aug 2012, American Journal of Immunology, pp. 65-70.
Link to Article
- Oleic Acid, deglycosylated vitamin D-binding protein, nitric oxide: a molecular triad made lethal to cancer. Ruggiero M, Ward E, Smith R, Branca JJ, Noakes D, Morucci G, Taubmann M, Thyer L, Pacini S. Jul 2014, Anticancer Research, pp. 3569-3578.
Link to Article